The signal transducer and activator of transcription 5 (Stat5) has been shown to cooperate with some nuclear receptors. However, an interaction has never been demonstrated with the androgen receptor (AR). Given that the PRL-inducible protein/gross cystic disease fluid-15 (PIP/GCDFP-15) is both a PRL-controlled and an androgen-controlled protein, we used its promoter region to investigate the potential interaction between Stat5 and androgen receptor. Dihydrotestosterone or PRL alone slightly modulated or did not modulate the luciferase activity of all reporter gene constructs. In contrast, a maximal increase was observed using the –1477+42 reporter gene construct after exposure to both dihydrotestosterone and PRL. The requirement of half-site androgen-responsive elements and two consensus Stat5-binding elements, Stat5#1 and Stat5#2, was determined by site-directed mutagenesis. Activated Stat5B binds with a higher affinity to Stat5#2 than to Stat5#1. Stat5AΔ749 and Stat5BΔ754 mutants demonstrated that the Stat5 trans-activation domain is involved in the hormonal cooperation. The cooperation depends on the PRL-induced phosphorylation on Tyr694 in Stat5A and Tyr699 in Stat5B, as demonstrated using the Stat5AY694F and Stat5BY699F proteins. The use of AR Q798E, C619Y, and C784Y mutants showed that transactivation, DNA-binding, and ligand-binding domains of AR are essential. Our study thus suggests a functional cooperation between AR and Stat5. (Molecular Endocrinology 16: 1696–1710, 2002)
SIGNAL TRANSDUCERS and activators of transcription (Stat) and nuclear steroid receptors are distinct transcription factor families that mediate cellular responses to diverse stimuli. Stat family members are latent cytoplasmic transcription factors identified as primary effector molecules for the cytokine/GH/ PRL receptor superfamily (reviewed in Ref. 1). They are activated by the receptor-associated Janus kinase via a process involving the phosphorylation of a single Stat tyrosine residue that induces dimerization of the Stat proteins through their Src homology 2 domain, followed by Stat translocation to the nucleus, binding to specific DNA enhancer elements, and activation of target gene expression (2–4). Seven mammalian Stat proteins (Stat1, -2, -3, -4, -5A, -5B, and -6) have been identified to date. Stat5 was originally identified in the mammary gland of lactating animals as mammary gland factor, a transcription factor that is activated by PRL (5–7). It is now recognized to be activated by many cytokines, including GH, as well as several other ILs and growth factors (8, 9). Stat5A and Stat5B are two highly related proteins that share 96% sequence homology and are encoded by two separated, but clustered, genes (10). These proteins differ in their carboxyl-terminal region, which is involved in trans-activation and DNA-binding activities (11). Both Stat5 activate target gene expression via Stat5 response elements, although with some differences in their DNA binding specificity (12, 13). Differences observed in the phenotype of Stat5A- and Stat5B-deficient mice, especially concerning GH and PRL signaling pathways, are due to differences in the level of expression, rather than an isotype-specific function. However, the loss of additional functions associated with GH, PRL, and IL-2 in mice deficient in both Stat5A and Stat5B indicates that Stat5 genes are often functionally redundant (14–17).
Nuclear steroid receptors, which include those for sex steroid hormones [i.e. progestins, estrogens (18), androgens (AR)] as well as those for adrenal steroid hormones (i.e. glucocorticoids (GR) and mineralocorticoids], are ligand-inducible transcription factors belonging to the nuclear receptor superfamily. They contain conserved domains that function in DNA binding, ligand binding, transcriptional activation, and dimerization (reviewed in Refs. 19 and 20).
In addition to the relatively well characterized Stat-Stat interactions (21), the Stat proteins interact with other transcription factors and nuclear molecules, including Sp1 (22); nuclear factor-κB (23); p300/cAMP response element binding protein (CREB) (24–27)-binding protein; peroxisome proliferator-activated receptors α, γ, and δ; thyroid hormone receptor (28, 29); and GR (26, 30). One of the best characterized models of Stat5 association with other transcription factors is the cross-talk between GR and Stat5 on the β-casein gene promoter. Stat5-dependent β-casein gene transcription is enhanced by the protein complex formed of Stat5, bound to its consensus DNA-binding site (TTCnnnGAA) and GR (31, 32).
Both Stat5A and Stat5B can confer the PRL response to the β-casein gene promoter, acting as homodimers in combination with GR in mammary epithelial cells (10, 32, 33). The association between GR and Stat5 has also been observed in tissue extracts prepared at all stages of mammary gland development, thus confirming the physiological relevance of this synergistic mechanism (32). The amino-terminal trans-activation function region-1 domain of GR is required for such a transcriptional synergy (33), whereas the C-terminal trans-activation domain of Stat5 is not necessary (33, 34). Although mineralocorticoid receptors, progestin receptors, and estrogen receptors synergize with Stat5 in the induction of β-casein gene transcription, cooperation has never been determined between AR and Stat5 (35, 36).
All genes encoding milk proteins identified to date (i.e. αS1-casein, β-casein, whey acidic protein, and β-lactoglobulin) contain at least one Stat5-binding site (for a review, see Ref. 37). The regulation of the gene encoding the PRL-inducible protein/gross cystic disease fluid-15 (PIP/GCDFP-15) protein was the first demonstrated regulation of gene expression by PRL in human target cells (38). PIP/GCDFP-15 is a glycoprotein secreted by the mammary gland and various apocrine glands as well as benign and malignant human breast tumors (39). The presence of a high concentration of the protein in breast fluid indicates a good prognosis in a subset of breast carcinomas (40, 41). A recent report also found that this protein is a novel aspartyl proteinase that might play a role in the proteolysis associated with invasive breast cancer lesions (42). In human breast cancer cell lines, its expression is increased by dihydrotestosterone (DHT), dexamethasone (DEX) (a synthetic glucocorticoid), PRL, and other cytokines (e.g. IL-1α, IL-4, IL-6, and IL-13) and is down-regulated by 17β-estradiol (38, 43–46). Furthermore, human GH acts in synergism with DEX or DHT to stimulate its gene expression (38, 43, 47).
The present study was thus designed to investigate the potential interaction between AR and Stat5A or Stat5B by using the PIP/GCDFP-15 gene promoter as a model. We report the cloning of 2188 bp of the 5`-flanking region of the PIP/GCDFP-15 gene and the location of two functional half-androgen response elements (1/2AREs) and two functional consensus Stat5-binding sites. The synergistic stimulatory action of DHT and PRL, a hormone structurally and functionally similar to GH, on PIP/GCDFP-15 gene transcription requires a functional interaction between activated Stat5 and activated AR, showing for the first time a cross-talk between Stat5 and AR signaling pathways.
RESULTS
Features of the 5`-Flanking Region of the Human PIP/GCDFP-15 Gene
The 5`-flanking region of the PIP/GCDFP-15 gene located from —898 to —1 contains the following cis-regulatory elements: a classical TATA box, a CAAT box, and two 1/2AREs (47). The present analysis of the 2188 nucleotides 5`-upstream flanking region from the transcription initiation site revealed the presence of two other potential 1/2AREs, located from —1325 to —1320 (1/2ARE#1: TGTTCT) and from —1276 to —1271 (1/2ARE#2: AGAACA), as well as two consensus DNA-binding sites for Stat5, located from —1230 to —1222 (Stat5#1: TTCaaaGAA) and from —142 to —134 (Stat5#2: TTCttaGAA; Fig. 1).
Deletion Mapping of the (—2188+267)-Promoter Region That Confers the Hormonal Response to the Human PIP/GCDFP-15 Gene
As a first step toward identifying specific cis-acting DNA sequences mediating the effects of PRL and DHT on human PIP/GCDFP-15 gene promoter activity, luciferase reporter gene constructs under the control of various 5`-deletion fragments of the upstream promoter region were used (Fig. 2B). Each of the seven reporter gene constructs was transfected into human ZR-75-1 cells together with expression plasmids for mouse PRL receptor (PRLR), human AR, and either mouse Stat5A or Stat5B. The transfected cells were incubated for 24 h in the presence of 5 μg/ml ovine PRL (ovPRL), 10 nM DHT, or a combination of both or were left untreated. DHT or ovPRL alone caused a minor effect, if any, on luciferase activity of promoter constructs (Fig. 2B). On the other hand, a simultaneous 24-h exposure to DHT and ovPRL increased luciferase activity of the 1477WT (—1477+42) construct by 6- and 12-fold in cells transfected with Stat5A and Stat5B, respectively. Progressive 5`-truncation from —1477 to —1267 or —708 decreased by 75–90% the synergistic stimulatory action of DHT and PRL in cells cotransfected with either Stat5A or Stat5B (Fig. 2B). Interestingly, the region from —1477 to —708 contains two sequence motifs related to half-sites of classical ARE and the Stat5#1 DNA-binding site (Figs. 1 and 2A). Luciferase activity of pGL3-basic vector mock was similar in cotransfected ZR-75-1 cells treated or left untreated (data not shown). An increase in basal activity of the PIP/GCDFP-15 promoter was detected with certain truncation mutants, suggesting the presence of negative response elements that may be involved in the hormonal responsiveness. An in silico analysis of the promoter indicated the presence of only a putative negative glucocorticoid response element (GRE) previously described in the promoter of the mouse mammary tumor virus (AGGATGT) (48). This element is located from —1434 to —1428 in the PIP/GCDFP-15 promoter. Using transient transfection assays, we studied the impact of its deletion on PIP/ GCDFP-15 promoter transcriptional activity. The deletion of this putative site did not affect the transcriptional activity (data not shown). The next experiment confirmed that both PRLR and AR are required to mediate the synergistic action of DHT and PRL on the PIP/GCDFP-15 gene promoter (Fig. 3).

Fig. 1. Nucleotide Sequence of the 5`-Flanking Region of the Human PIP/GCDFP-15 Gene
The 5`-flanking region of the gene is numbered —1 to —2188. The +1 indicates the transcription start site. The initiation codon (ATG) as well as the putative TATA box and CAAT box are boxed. The four putative half-AREs are in bold and underlined, and the two putative Stat5-binding sites are in bold and double underlined.
Potential Role of Stat5- and 1/2ARE-Binding Sites in the Synergistic Action of DHT and PRL
To decipher the mechanism underlying the synergistic action of DHT and PRL, we studied the roles of Stat5#1, Stat5#2, 1/2ARE#1, and 1/2ARE#2 (Fig. 4). We focused our investigation on these two 1/2ARE because of the marked decrease in the synergistic effect of DHT and PRL on the transcriptional activation of the PIP/GCDFP-15 gene after the truncation from —1477 to —1267 (Fig. 2).
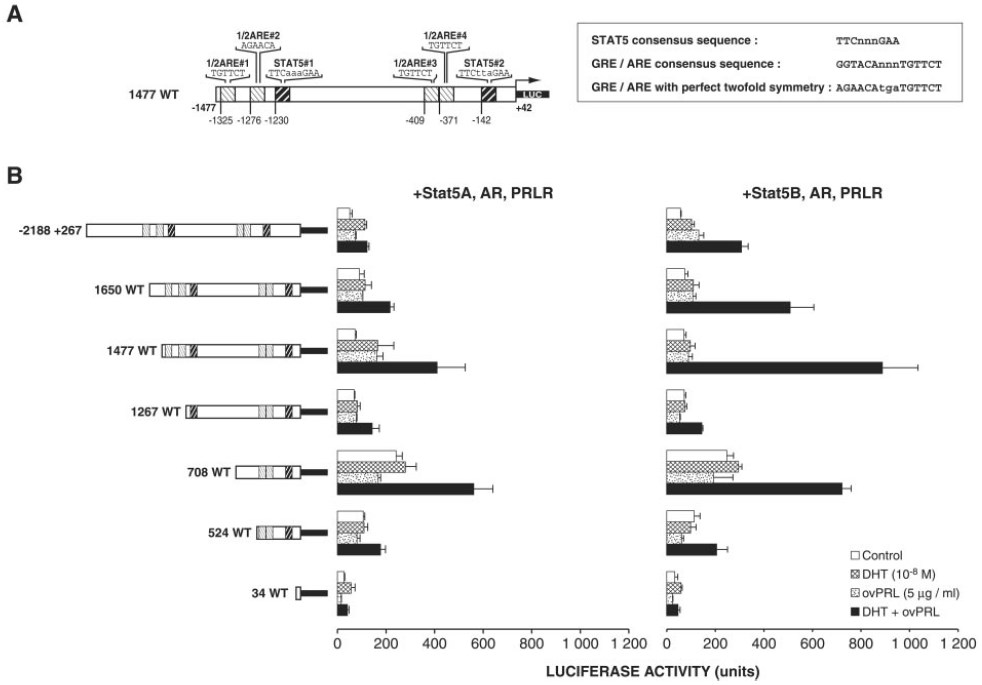
Fig. 2. Deletion Mapping of the Promoter Region that Confers the Hormonal Response to the PIP/GCDFP15 Gene
A, Schematic representation of the (—1477+42)-PIP/GCDFP-15 promoter fragment. The four potential 1/2AREs and the two potential Stat5-binding sites of the 1477WT fragment of the upstream PIP/GCDFP-15 promoter region are indicated. The sequences for the consensus GRE/ARE and the consensus Stat5-binding site are in capital letters, and spacer nucleotides between half-sites are in lowercase letters. B, Deletion mapping of the (—2188+267) promoter region that confers the hormonal response to the human PIP/GCDFP-15 gene. A family of seven 5`-deletion fragments was generated by PCR. These fragments were inserted in the sense orientation into the plasmid pGL3-basic(Luc). The 5`-end of each fragment is given to the relative transcription initiation site of the gene. ZR-75-1 cells were cotransfected with 0.25 μg reporter plasmid, 0.25 μg expression vector encoding mouse PRLR, 0.25 μg expression vector encoding human AR, 0.25 βg expression vectors encoding either mouse Stat5A or Stat5B, and 0.1 μg cytomegalovirus-β-galactosidase for each well. One day after transfection, the cells were grown in serum-free medium for 24 h in the presence of 5 μg/ml ovPRL, 10 nM DHT, or both hormones or were left untreated before harvesting and assayed for luciferase expression as described in Materials and Methods. Data are expressed as the mean ± SEM of triplicate dishes.
A point mutation of putative 1/2ARE#1 decreased by 74% and 75% the level of the stimulatory effect of DHT and PRL on luciferase activity of the 1477WT construct detected in ZR-75-1 cells cotransfected with Stat5A or Stat5B, respectively, and a point mutation of 1/2ARE#2 decreased these levels by 89% and 66%, respectively (Fig. 4). Point mutations in both 1/2ARE#1 and 1/2ARE#2 [m1477(1/2ARE#1+1/2ARE#2) construct] decreased basal luciferase activity and abolished the stimulatory effect of DHT and PRL, indicating the importance of both binding sites for the synergistic action (Fig. 4).
On the other hand, a point mutation of putative Stat5#1 decreased by 79% and 56% the level of the stimulatory effect of DHT and PRL detected in ZR-75-1 cells cotransfected with Stat5A or Stat5B, respectively. Furthermore, Stat5#1 appeared to cooperate with either 1/2ARE#1 or 1/2ARE#2. Indeed, a mutation of Stat5#1 in combination with a point mutation in 1/2ARE#1, 1/2ARE#2, or both completely abolished the stimulation of DHT and PRL on luciferase activity in ZR-75-1 cells cotransfected with Stat5A (Fig. 4). In Stat5B cotransfected cells, a mutation of Stat5#1 with a mutation in 1/2ARE#1 or 1/2ARE#2 also diminished the synergistic action by 71% and 85%, respectively, whereas this effect was decreased by 92% when using the m1477(1/2ARE#1+1/2ARE#2+Stat5#1) reporter construct (Fig. 4).
As illustrated in Fig. 4, a point mutation of Stat5#2 alone or in combination with any mutation involving 1/2ARE#1, 1/2ARE#2, or both and the combined mutation of all four sites bolished the stimulatory effect of DHT and PRL on luciferase activity in ZR-75-1 cells cotransfected with Stat5A or Stat5B. This may reflect the importance of the proximal Stat5#2-binding site.

Fig. 3. Functional Synergy between AR and Stat5 Signaling Pathways
Cells were transiently cotransfected with the indicated constructs (at the same amounts as described in Fig. 2B) and treated as described in Fig. 2B. The total amount of DNA was adjusted to 1 μg/well using pcDNA3.
Stat5 Binds to Stat5#1 and Stat5#2 Consensus Sequences in the PIP/GCDFP-15 Promoter
To confirm the role of Stat5#1 and Stat5#2 consensus sequences in the synergistic action of DHT and PRL on transcriptional activation of the PIP/GCDFP-15 gene, we next examined whether Stat5 was able to bind to these sites (Fig. 5). For this, the COS7 cell line was used in electrophoretic mobility shift assays (EMSA) because this well established cell expression system is appropriate for high level, short-term protein expression (49). 32P-Labeled double-stranded oligonucleotides containing either Stat5#1 or Stat5#2 were incubated with nuclear extracts (10 μg) obtained from transiently transfected COS7 cells overexpressing AR, PRLR, and Stat5B. Cells were treated with ovPRL for 20 min, 10 nM DHT for 24 h, or both hormones or were left untreated. With the Stat5#1 plus 1/2ARE#2 and Stat5#2 probes, a protein-DNA complex was observed in nuclear extracts of cells treated with PRL or with DHT plus PRL. DHT treatment alone did not induce a detectable complex (Fig. 5A). The formation of the complex was weaker with the Stat5#1 plus 1/2ARE#2 probe. Whichever probe used, the intensity of the signal in nuclear extracts treated by DHT and PRL was similar to the intensity of the signal in nuclear extracts treated with PRL (Fig. 5A). As a result, a 24-h DHT pretreatment did not facilitate PRL-activated Stat5 DNA-binding activity. In a follow-up experiment we studied the effect of adding ovPRL for various time periods (0, 7, and 20 min and 1 and 24 h) after a 24-h DHT pretreatment of COS7 cells on the DNA binding activity of Stat5B (Fig. 5B). The duration of the ovPRL treatment did influence Stat5B DNA binding, as a protein-DNA complex was already detected 7 min after exposure to the hormone, its abundance peaked at 20 min, and a high level of protein-DNA complex remained after 24-h incubation. In all of these experiments, the presence of Stat5B protein in the complexes was confirmed by incubating the nuclear extracts with antiserum specific to Stat5B before the addition of probes. Similar results were found using nuclear extracts from COS7 cells overexpressing Stat5A, AR, and PRLR and treated with DHT plus PRL, although with weaker intensity of the binding signal (data not shown). The use of antiserum specific to Stat5A also confirmed the presence of Stat5A within the complexes activated by DHT and PRL (data not shown). In addition, antibodies against C-terminal AR did not supershift these complexes (Fig. 5A), nor did antibodies against N-terminal AR (data not shown). A complex was also formed with whole cell extracts from transiently transfected ZR-75-1 cells treated with PRL or DHT and PRL; however, the intensity of the binding signal was weaker (data not shown).
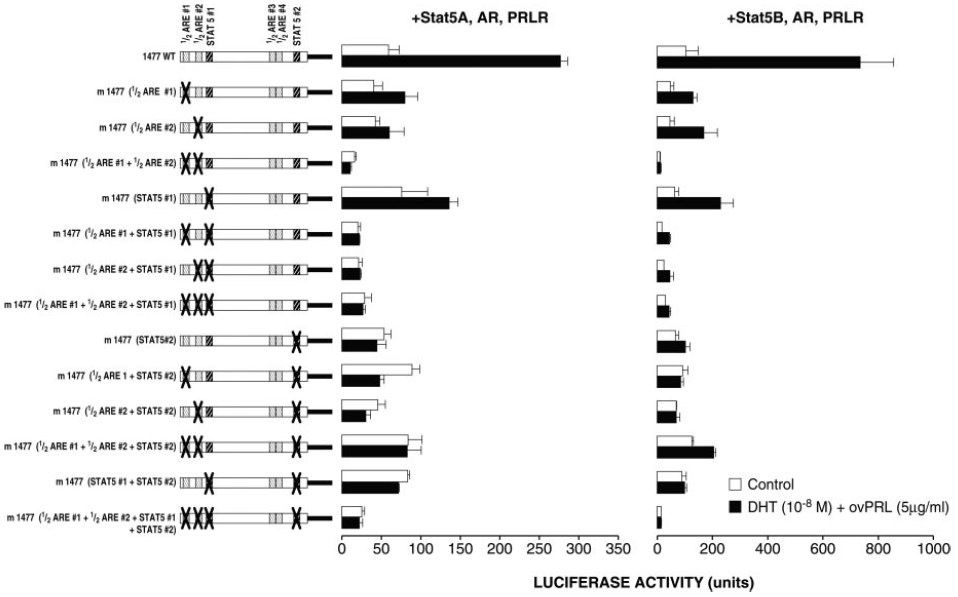
Fig. 4. Effect of Point Mutations Introduced into Putative 1/2-AREs and Stat5-Binding Sites of the 1477WT on the Synergistic Action of DHT and PRL
Experimental procedures were similar to those described in Fig. 2B, except that 1477WT and mutated versions were used.
Role of the trans-Activation Domain of Activated Stat5 in ZR-75-1 Cells
Stat5A and Stat5B differ in their C-terminal tails, a region that contains the major trans-activation domain of the Stat proteins (1, 11). Using carboxyl-terminally deleted forms of Stat5A and Stat5B (i.e. Stat5AΔ749 and Stat5BΔ754), which lack the major transcriptional activation domain region (but still retain their DNAbinding capacity and are able to be tyrosine phosphorylated) (11, 50), we investigated the role of the Stat5 trans-activation domain in the synergistic action of DHT and PRL on luciferase activity of the 1477WT reporter construct. As shown in Fig. 6, both truncated mutant proteins are impaired in their ability to activate luciferase activity in ZR-75-1 cells and HeLa cells, suggesting that the trans-activation domains of Stat5A and Stat5B are required for cooperation with the AR.
Stat5 proteins undergo tyrosine phosphorylation on a conserved tyrosine residue required for their homodimerization and translocation to the nucleus where they induce the transcription of cytokineresponsive genes (51, 52). We used Tyr→Phe mutants, Stat5AY694F and Stat5BY699F, to study the effect of tyrosine phosphorylation on PIP/GCDFP-15 gene promoter activity. Mutation of either Tyr694 in Stat5A or Tyr699 in Stat5B abolished 1477WT luciferase activity in ZR-75-1 cells and HeLa cells as well (Fig. 6). These data suggest that PRL-induced phosphorylation on Tyr694 in Stat5A and Tyr699 in Stat5B was essential to establish the functional cooperation between Stat5 and AR on the transcriptional activation of the PIP/GCDFP-15 gene by DHT and PRL.
Effect of Stat5AΔ749 and Stat5BΔ754 on Transcriptional Activation of the PIP/GCDFP-15 Gene by Wild-Type Stat5A and Stat5B
Stat factors lacking the COOH-terminal trans-activation domain can act as dominant negative mutants, presumably by forming inactive heterodimers with wild-type Stat (11, 50, 53). We next studied whether Stat5AΔ749 and Stat5BΔ754 were able to block the trans-activation function of wild-type Stat5A and Stat5B on PIP/GCDFP-15 reporter gene activity in ZR-75-1 cells. As shown in Fig. 7, simultaneous exposure to DHT and PRL increased luciferase activity of the 1477WT construct by 2.6- and 5.7-fold in ZR-75-1 cells cotransfected with Stat5A or Stat5B, respectively. Addition of increasing concentrations of either Stat5AΔ749 or Stat5BΔ754 expression vector abolished the stimulatory effect mediated by wild-type Stat5A or Stat5B (Fig. 7). Our findings demonstrate that both deletion mutants were able to exert a dominant negative effect on the trans-activation function of wild-type Stat5A and Stat5B.
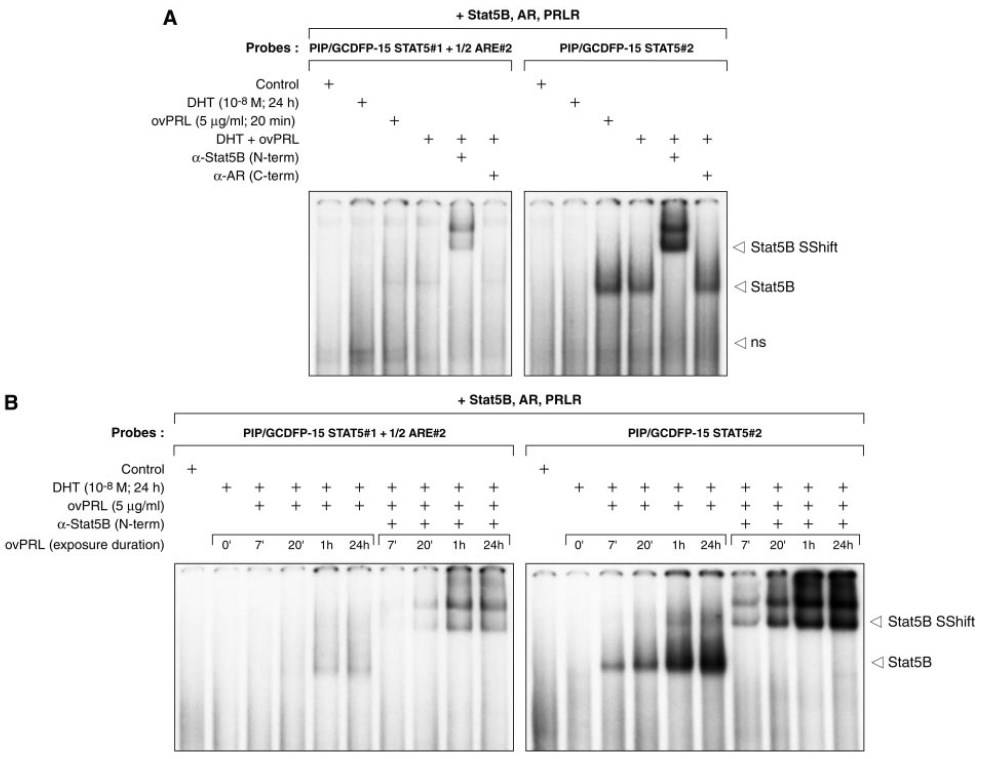
Fig. 5. STAT5b Binds to STAT5#1 and STAT5#2 Consensus Sequences in the PIP/GCDFP15 Promoter
A, Binding of Stat5B to Stat5#1+1/2ARE#2 and Stat5#2 consensus sequences in the PIP/GCDFP-15 gene promoter. 32P-Labeled double-stranded oligonucleotides (80,000 cpm) containing putative Stat5 sites (Stat5#1 and Stat5#2) were incubated with nuclear extracts (10 μg) obtained from COS7 cells overexpressing AR, PRLR, and Stat5B and treated with 5 μg/ml ovPRL for 20 min, 10 nM DHT for 24 h, or both hormones. When used, specific antisera to Stat5B or C-terminal AR (2 μl/reaction; 200 μg/100 μl) were incubated with nuclear extracts before the addition of the probes (see Materials and Methods for further details). ns, Nonspecific protein binding to the probes. B, Effect of PRL after a 24-h DHT pretreatment on the DNA-binding activity of Stat5B. Twenty-four-hour DHT-pretreated nuclear extracts were prepared at various times (0, 7, and 20 min, and 1 and 24 h) after the addition of 5 μg/ml ovPRL and subjected to EMSA as described in A. ns, Nonspecific protein binding to the probes.
Role of trans-Activation, Ligand-Binding, and DNA-Binding Domains of AR in the Synergistic Effect of DHT and PRL
The structure of AR, a member of the nuclear receptor superfamily, is divided into domains that function in DNA-binding, ligand-binding, trans-activation, and dimerization (54). To discriminate the potential role of the DNA-binding, ligand-binding, and trans-activation domains of AR in the cooperation with Stat5A or Stat5B, the mutation C619Y, C784Y, or Q798E was introduced into the full-length AR expression vector, respectively (55–57). In cells overexpressing wild-type AR and Stat5B, exposure to DHT and PRL caused a 9-fold increase in the luciferase activity of the 1477WT reporter gene construct (Fig. 8). Both C619Y and C784Y mutations abolished the stimulatory effect induced by DHT and PRL, whereas the trans-activation response observed using Q798E was significantly lower than that obtained with the wild-type AR. Similar findings were found with Stat5A and these three AR mutants (data not shown). As revealed by Western blot analyses, altered functions of all AR mutants were not due to lower AR protein expression levels compared with that of wild-type AR protein (data not shown). In addition, in the presence of AR, DHT increased by 5.2-fold the luciferase activity of the androgenresponsive reporter construct [pTK-4XARE/GRE-Luc], whereas this stimulatory effect was decreased in cells overexpressing the AR C619Y, C784Y, and Q798E mutants (Fig. 8). Together these data suggest that DNA-binding, ligand-binding, and transcription activation domains of AR were essential for optimal functional cooperation with Stat5 isoforms in the synergistic action of DHT and PRL on transcriptional activation of the PIP/GCDFP-15 gene.
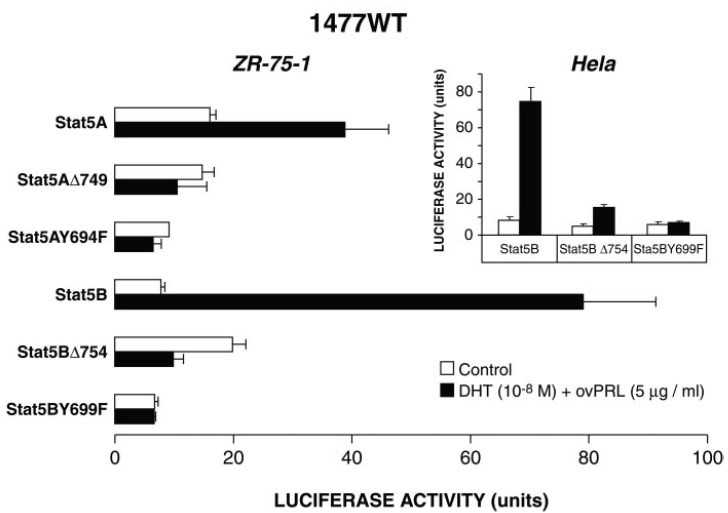
Fig. 6. Effects of Stat5AΔ749, Stat5BΔ754, Stat5AY694F, and Stat5BY699F on the Synergistic Action of DHT and PRL
Experimental procedures were similar to those described in Fig. 2B, except that Stat5 mutants were overexpressed in ZR-75-1 cells. In HeLa cells, 1477WT (250 ng) was cotransfected with expression vectors encoding AR (75 ng), PRLR (250 ng), Stat5B or Stat5B mutant forms (75 ng), and cytomegalovirus-β-galactosidase (100 ng) for each well.
DISCUSSION
There is considerable evidence of Stat interaction with other transcription factors bound to neighboring sites in upstream segments of various target genes (for reviews, see Refs. 3 and 58). The Stat proteins can participate in transcriptional activation through four distinct mechanisms. 1) They bind to their own DNAbinding site to directly enhance transcription. 2) They form a transcriptional complex with a non-Stat transcription factor and bind to a Stat DNA-binding site or 3) to a non-Stat DNA-binding site to trigger transcription (example of Stat5 and GR to activate the β-casein gene). 4) They cooperate with a non-Stat transcription factor(s) to activate transcription by binding to clustered independent DNA-binding sites (for examples, Sp1 with Stat1 or Stat3 to activate the intracellular adhesion molecule-1 gene or the CCAAT/enhancer binding protein δ gene, respectively) (for reviews, see Refs. 3 and 59).
Using various luciferase constructs containing the 5`-flanking region of the PIP/GCDFP-15 gene, we showed for the first time that PRL-activated Stat5 and DHT-activated AR stimulated target gene transcription in a synergistic way. The synergistic action of PRL and DHT on transcriptional activity of the PIP/GCDFP-15 gene promoter was also observed both with T47-D human breast cancer cells and HeLa human cervical cancer cells (data not shown). Taken together, our results suggest a model that is similar in certain ways to that previously reported for the δ-casein promoter, especially in that it calls for the cooperation of different signaling pathways.
Our data indicate that both the Stat5 trans-activation domain and the AR trans-activation domain participated in the functional synergy involving Stat5 and AR signaling pathways. Using carboxyl-truncat d Stat5AΔ749 and Stat5BΔ754 mutants, h ch lack the major transcriptional activation domain region but still retain their DNA-binding capacity and are able o be tyrosine phosphorylated), we documented here that the trans-activation domain of Stat5 was crucial or the transcriptional synergy. In contrast, it was repo ted that the strong trans-activation domain in GR can sup lement the relatively weak trans-activation domain in Stat5 for the synergistic action of DEX and PR on transcriptional activation of the δ-casei ge e (33, 34). Others showed that the COOH-terminal trans-act vat on domain of Stat5B is critical for transcriptional r pr ssion of the interferon regulatory factor-1 gene, inhibitory cross-talk with peroxisome proli erator-activated receptor-α, and transcriptional activa ion of the δ-casein gene in the presence of PRL alone (11, 2 , 60). In addition, and in accordance with previous obse v tions (11, 50, 53), we found that both Stat5AΔ749 and Stat5BΔ754 mutants exerted a dominant n gative effect on the trans-activation function of Stat5 and Stat5B, presumably by forming inactive heterodim rs with wild-type Stat5 or nonfunctional homodimers th t bound more persistently and strongly to DNA. Initia studies revealed that tyrosine phosphorylation of a specific residue of Stat5 is essential for dimeriza ion and trans-activation (51, 52). The present study de ons rated the requirement of the PRL-induced phosphorylation on Tyr694 in Stat5A and Tyr699 in Stat5B in the synergistic effect of DHT and PRL on transcriptional activation of the PIP/GCDFP-15 gene.
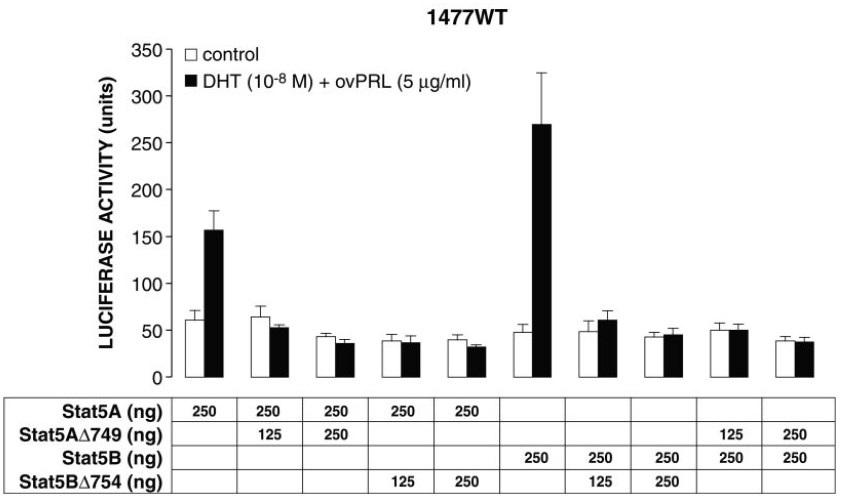
Fig. 7. Effects of Stat5AΔ749 and Stat5BΔ754 on the 1477WT Transcriptional Induction by Synergistic Action of DHT and PRL via Wild-Type Stat5A and Stat5B
The experimental procedures were similar to those described in Fig. 2B, except that wild-type Stat5 proteins and Stat5 mutants were overexpressed as indicated. The total amount of DNA was adjusted to 1.5 μg/well using pcDNA3.
Use of the AR Q798E mutant demonstrated that the transcriptional activation domain of AR was essential in the cooperative mechanism. The Q798E mutation is in the ligand-binding domain and does not affect any ligand-binding property, but alters the trans-activation function (55). Involvement of the trans-activation function region-1 domain of GR in the functional cooperation between GR and Stat5 was similarly reported (33). Finally, results with the AR C784Y mutant confirmed that ligand binding was essential for functional synergism of androgen and PRL (the missense mutation completely abolishes ligand-binding and transactivation functions of AR) (56). Collectively, our findings clearly show that Stat5A and Stat5B must be transcriptionally active to cooperate with DHT-activated AR.
Despite the importance of glucocorticoids in milk protein gene expression, most of these genes contain 1/2GRE/ARE and do not contain consensus GRE/ARE (GGTACAnnnTGTTCT), a common high affinity DNAbinding target that can be trans-activated in vitro by AR, progestin receptors, and GR (61). A computer analysis indicated that the —1477+42 fragment of the upstream PIP/GCDFP-15 promoter region (1477WT) contained four 1/2AREs as well as two consensus Stat5-binding sites. Point mutations of the 1/2AREs and Stat5-binding sites led to the conclusion that the distal promoter region containing 1/2ARE#1, 1/2ARE#2, and Stat#1 as well as the proximal promoter region containing Stat5#2 were necessary for mediation of the DHT and PRL synergy. The integrity of both 1/2AREs and Stat5-binding sites was required for PIP/GCDFP-15 gene responsiveness to DHT and PRL. This is in accordance with what was reported about Stat5 and GR transcriptional synergy at the β-casein promoter: glucocorticoid-dependent transcriptional synergy with PRL occurs through GR binding to 1/2GRE, facilitated by the protein-protein interactions of Stat5 and GR (34). In contrast, in an earlier study Stocklin et al. (31) reported that GR cooperates with Stat5 in a way that is independent of a 1/2GRE: Stat5-dependent transcription was enhanced by protein complexes formed between Stat5, bound to its consensus DNA-binding site, and GR (31). Unfortunately, by using coimmunoprecipitation studies (data not shown) and EMSA supershift analysis (Fig. 6), we failed to detect any complexes containing Stat5 and AR. The protein complexes containing activated Stat5B bound to either Stat5#1 probe or Stat5#2 probe were not supershifted by anti-AR antibodies (Fig. 5A). This confirms that the physical interaction between AR and Stat5, if it exists, may be difficult to observe. However, as previously mentioned, functional responses with mutations of the PIP/GCDFP-15 promoter showed that the 1/2AREs were required for synergistic stimulation by androgens and PRL. This suggests that AR binding was involved. Because weak AR binding to 1/2AREs is difficult to detect in vitro, although functionally significant, we used a DNA binding-deficient AR mutant (C619Y) in transfection experiments to distinguish between DNA-dependent from DNA-independent mechanisms. The C619Y mutation, near the cysteines coordinating zinc in the DNA-binding domain, leads to an AR form that is transcriptionally inactive and unable to bind DNA (57). Our findings with this mutant confirmed that AR binding to DNA was involved in the synergistic stimulation of DHT and PRL.

Fig. 8. Role of the trans-Activation, Ligand-Binding, and DNA-Binding Domains of AR in the Synergistic Action of DHT and PRL
A, pTK-4XARE/GRE-Luc (250 ng) was used as an androgen-responsive reporter construct and was cotransfected into HeLa cells with AR or AR mutant forms (75 ng) and cytomegalovirus-β-galactosidase (100 ng) for each well. 1477WT (250 ng) was cotransfected with expression vectors encoding Stat5B (75 ng), PRLR (250 ng), AR or indicated AR mutant forms (75 ng), and cytomegalovirus-β-galactosidase (100 ng) for each well. B, Transfection conditions in ZR-75-1 cells were as described in Fig. 2B. pTK-4XARE/GRE-Luc was used as the androgen-responsive reporter construct (250 ng), and mtAR C784Y (250 ng) was used as an AR mutant form. The transfected cells were grown in serum-free medium for 24 h in the presence of 10 nM DHT or 5 μg/ml ovPRL and 10 nM DHT or were left untreated.
Although Stat5A and Stat5B show approximately 90% amino acid sequence identity, their binding specificity for the DNA-binding site in target genes can differ (12–15, 32, 62). Our results indicate that, indeed, Stat5B preferentially formed complexes with both consensus Stat5 elements (TTCnnnGAA) of the PIP/GCDFP-15 promoter region (Fig. 5A and data notshown). In support of this observation, stimulation of the transcriptional activity of the 1477WT PIP/GCDFP-15 reporter gene construct by DHT and PRL was always higher in cells cotransfected with Stat5B than in cells cotransfected with Stat5A. Interesting y, our preliminary results obtained in ZR-75-1 showed that, in contrast to DHT, a 24-h exposure to dexam thasone (100 nM) alone was able to increase the luciferase activity of the 1477WT construct in cells overexpr ssing GR and Stat5A or Stat5B, and this effect was stronger in T47-D human breast cance cells. Moreover, in both cell lines a synergist c effect was observed between DEX and PRL in cells ov rexpressing Stat5A and GR, but not in those cotransf cted with Stat5B and GR (data not shown). This mig t well un erline a functional difference between Stat5A a d Stat5B in mechanisms of transcriptional e ulation by glucocorticoids and androgens in combination with PRL on the PIP/GCDFP-15 promoter.
The stimulation of PIP/GCDFP-15 gene expression by DHT and PRL via activated Stat5A or Stat5B and DHT-activated AR may well have physiological or pathological consequences, or both. In this regard, although PRL favors or induces mammary gland tumor formation in rodent models (63) and stimulates the proliferation of mammary tumor cell lines (64, 65), its role remains to be clarified in humans. On the other hand, androgens or androgenic compounds can have beneficial effects on breast cancer growth in women (66–71). Combined with an antiestrogen, they lead to a higher response rate and a longer time to disease progression than an antiestrogen alone (70, 71). They also induce an objective remission after failure of antiestrogen therapy and hypophysectomy. Together this indicates that benefits gained from androgen therapy rely not only on suppression of pituitary gonadotropin secretion, but also, at least in part, on a direct effect of androgens on tumor growth. This is supported by the presence of AR in many human breast cancers (72–75). In humans and some other primates, adrenals secrete large amounts of dehydroepiandrosterone (DHEA) and DHEA sulfate, steroid precursors that are converted into potent androgens and estrogens in peripheral tissues by 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase, 17β-hydroxysteroid dehydrogenase, 5α-reductase, and aromatase (76–78). It is also of interest to note that intracrine formation of potent androgens from DHEA in breast cancer cells as well as in normal epithelial cells of the human mammary gland can be regulated by cytokines (for a review, see Ref. 79). In vivo experiments showed that DHEA inhibits the development of 7,12-dimethylbenz(a)an-thracene-induced mammary tumors in rats and growth of ZR-75-1 breast cancer xenografts in ovariectomized nude mice (80, 81). In addition, the antiproliferative action of androgens in human breast cancer cells is well supported by several in vitro studies. Androgens and estrogens have antagonistic effects in several breast cancer cells (44, 78, 82–86). In ZR-75-1 cells, androgens markedly decrease ER expression, whereas they increase the length of the cell cycle, suggesting that these effects may explain the benefits of androgens in breast cancer therapy (87, 88). The inhibitory action of androgens on ZR-75-1 cell proliferation could also be due to down-regulation of the expression of Bcl-2, an oncoprotein that blocks programmed cell death and extends B cell survival (84). In human breast cancer CAMA-1 cells, DHT was demonstrated to cause an accumulation of the cyclindependent kinase inhibitor p27Kip1 and increase the proportion of cells in the G1 phase of the cell cycle. Thus, inhibition of CAMA-1 cell growth by androgens may be mediated at least in part by inactivation of the cyclin E-cyclin-dependent kinase-2 complexes by p27Kip1 (85).
In ZR-75-1 cells, androgens inhibit estrogeninduced cathepsin D and pS2 expression levels and regulate the secretion of apolipoprotein D and PIP/GCDFP-15 proteins (44, 89). The PIP/GCDFP-15 protein, which is secreted by the mammary gland and various apocrine glands as well as benign and malignant human breast tumors, is indeed increasingly recognized as a valuable marker for breast cancer (39, 90, 91). For example, a high concentration of this glycoprotein in breast fluid is indicative of good prognosis in subsets of breast carcinomas (40, 41). This might be correlated to the observation that steroids as well as IL-1α, IL-4, and IL-13 have opposite effects on the regulation of PIP/GCDFP-15 expression and ZR-75-1 cell proliferation (44–46, 92, 93). On the other hand, a recent report demonstrated that the PIP/GCDFP-15 protein produced by tumoral cells is a retrovirus-like aspartyl protease and might facilitate cell invasion by cleaving the extracellular matrix scaffold between cells, thus detaching cell membranes from adhesion sites (42). One of the most relevant in vitro functions of the PIP/GCDFP-15 protein reported to date is its strong inhibitory effect on the T lymphocyte apoptosis induced by sequential activation of CD4 and the T cell receptor (94). Furthermore, its marked increase in response to various cytokines, especially IL-4 and IL-13 (45, 46), might modulate the activity of breast tumorinfiltrating CD4+ T cells. Although its role in lymphocyte dysfunction in vivo remains to be elucidated, the ability of the PIP/GCDFP-15 protein to interfere with the signaling pathways of T lymphocytes is interesting in regard to the behavior of tumor-infiltrating lymphocytes in breast cancers that express the PIP/GCDFP-15 protein.
MATERIALS AND METHODS
Plasmids
A genomic DNA fragment corresponding to —2188 to +267 of the human PIP/GCDFP-15 gene served as a template in PCR reactions to create a series of six 5`-deletion fragments with a common 3`-end (+42). The sense primers and a single antisense primer incorporated unique restriction sites. The amplified fragments were cloned into the promoterless luciferase vector pGL3-Basic (Promega Corp., Madison, WI). Point mutations of the 1/2ARE and the Stat5-binding sites were generated in the —1477 to +42 promoter fragment (1477WT) using the QuikChange site-directed mutagenesis protocol of Stratagene (La Jolla, CA). The primers used for the site-directed mutagenesis were: mouse 1/2ARE#1, 5`-CCTCACAAGCAGTaTTCTTGAGTGGATAGG-3` (sense) and 5`-CCTATCCACTCAAGAAtACTGCTTGTGAGG-3` (antisense); mouse 1/2ARE#2, 5`-GCATGTGATCATGAGAAtAGCTGAAGTTCTGAG-3` (sense) and 5`-CTCAGAACTTCAGCTaTTCTCATGATCACATGC-3` (antisense); mouse Stat5#1, 5`-GTGTCCAATgCAAAGcAAAGAGAAGG-3` (sense) and 5`-CCTTCTCTTTgCTTTGcATTGGACAC-3` (antisense); and mouse Stat5#2, 5`-GCTGAGTGTGATTTTTgCTTAGcAAAACAAACTTTGGG-3` (sense) and 5`-CCCAAAGTTTGTTTTgCTAAGcAAAAATCAACACTCAGC-3` (antisense). Human AR was cloned into the pCMV-Neo-expressing vector. The expression vectors pcDNA1-PRLR, pXM-mouse Stat5A, pXM-mouse Stat5B, and the carboxyl-terminally deleted mutants Stat5AΔ749 and Stat5BΔ754, lacking the major transcriptional domain, were provided by Bernd Groner (Georg-Speyer-Haus, Biomedical Research Institute, Germany). The point mutants Stat5AY694F and Stat5BY699F, and the AR C619Y and Q798E mutants were generated by site-directed mutagenesis. pTKLuc containing four copies of the ARE/GRE element has been described previously (95).
Cell Culture
All media and supplements for cell culture were obtained from Sigma (St. Louis, MO), except for fetal bovine serum (FBS), which was provided by HyClone Laboratories, Inc. (Logan, UT). ZR-75-1 cells, HeLa cells, and COS7 cells were obtained from the American Type Culture Collection (Manassas, VA). ZR-75-1 cells were grown in phenol red-free RPMI 1640 medium supplemented with 1 nM 17β-estradiol, 2 mM L-glutamine, 1 mM sodium pyruvate, 12 mM HEPES, 100 IU/ml penicillin, 50 μg/ml streptomycin sulfate, and 10% FBS. COS7 cells and HeLa cells were grown in phenol red-free DMEM/high glucose medium containing 2 mM Lglutamine, 50 μg/ml streptomycin sulfate, 44 mM NaHCO3, and 5% FBS.
Transient Transfection Assays
ZR-75 cells were plated at 80,000 cells/well in 12-well plates. The following day, they were transfected with 0.25 μg luciferase reporter plasmid, 0.25 μg expression vectors encoding receptors, 0.25 μg Stat5 expression vectors, and 0.1 μg cytomegalovirus-β-galactosidase for each well using the Ex-Gen 500 reagent (MBI Fermentas, Inc., Amherst, NY) according to the manufacturer’s instructions. One day after transfection, the cells were incubated for various duration of time (see the figure legends for detail) in the presence of 5 βg/ml ovPRL, 10 nM DHT, 100 nM DEX, or a combination of two hormones (PRL plus DHT or PRL plus DEX) or were left untreated. The cytomegalovirus-β-galactosidase expression vector was included in all transfections to monitor transfection efficiency using β-galactosidase chemiluminescent reporter gene assay from Tropix, Inc. (Bedford, MA). Luciferase activities were measured using the Luciferase Assay System (Promega Corp., Madison, WI), were normalized to the β-galactosidase activities and expressed as arbitrary units. Data are expressed as the mean ± SEM of triplicate dishes and are representative of at least three independent experiments.
Preparation of Mininuclear Extracts from COS7 Cells
COS7 cells were grown in 100-mm culture dishes in phenol red-free DMEM supplemented with 5% charcoal dextrantreated FBS as described above. Upon reaching 70% confluence, cells were cultivated for 24 h in fresh serum-free medium, then transfected with 4 μg human AR, 4 μg mouse PRLR, and 4 μg mouse Stat5A or Stat5B. One day after transfection, the cells were treated with 5 μg/ml ovPRL, 10 nM DHT, or both in serum-free medium for the indicated times or were left untreated. Then they were washed twice with ice-cold PBS and scraped off the plates in cold PBS. Packed cells were resuspended in one packed cell volume of hypotonic buffer [10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM dithiothreitol, and 0.5 mM phenylmethylsulfonylfluoride] and allowed to swell on ice for 15 min. Cells were then lysed by rapidly pushing them through a narrow-gauge hypodermic needle. Supernatants were collected after centrifugation at 4 C (12,000 × g for 5 min). The crude nuclear pellets were resuspended in a two thirds volume of a high salt buffer [20 mM HEPES (pH 7.9), 25% (vol/vol) glycerol, 1.5 mM MgCl2, 420mM NaCl, 0.2mM EDTA, 0.5mM dithiothreitol, and 0.5mM phenylmethylsulfonylfluoride], followed by incubation on ice with stirring for 30 min and then were centrifuged at 12,000 × g for 5 min. The protein concentration of nuclear extracts was determined by using the Bio-Rad Laboratories, Inc. (Hercules, CA) protein assay. Protein extracts were aliquoted and frozen at —70 C.
EMSAs
The sequences of double-stranded oligonucleotides containing potential DNA-binding sites for Stat5 are as follows: Stat5#1+1/2ARE#2, 5`-GTGATCATGAGAACAGCTGAAGTTCTGAGTGAGCAATGACATGAATGGTGTCCAATTCaaaGAAAAGAGAAGG-3`; and Stat5#2, 5`-GCTGACATTCTAGCTGAGTGTTGATTTTTTCttaGAAAAACAAACTTTGGGTCAACAAGGAAAGATCACG-3`. Ten micrograms of nuclear proteins were used per 20 μl reaction carried out in binding buffer [10 mM Tris-HCl (pH 7.5), 1 mM dithiothreitol, 25 mM KCl, and 10% (vol/vol) glycerol] containing 2 μg poly(deoxyinosine)-poly(deoxycytosine) [poly(dI)-poly(dC)] (Pharmacia Biotech, Piscataway, NJ) and 1 μg BSA. Nuclear extracts were first incubated with poly(dl)-poly(dC) and BSA at room temperature for 10 min. The binding reaction, initiated by adding a 32P 5μ end-labeled synthetic oligonucleotide probe (80,000 cpm), was conducted at room temperature for 20 min. Samples were then incubated on ice for 10 min. Reactions were separated on a 5% nondenaturing polyacrylamide (38:2, acrylamide/bis-acrylamide) gels in 0.5× Trisglycine-EDTA electrophoresis buffer, pH 8.3. When used, polyclonal antibodies (2 μl/reaction; 200 μg/100 μl) were included with the extracts on ice for 30 min before the addition of poly(dI)-(dC), BSA, and binding buffer. Anti-Stat5A antibodies (L-20), anti-Stat5B antibodies (N-20), and anti-AR antibodies (N-term and C-term) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Acknowledgments
We thank the members of the Laval University Medical Research Center Illustration Service for artwork.
Received July 20, 2001. Accepted February 27, 2002.
Address all correspondence and requests for reprints to: Dr. Jacques Simard, Cancer Genomics Laboratory, T3-57, Oncology and Molecular Endocrinology Research Center, Laval University Medical Center (CHUL), 2705 Laurier Boulevard, Sainte-Foy, Quebec, Canada G1V 4G2. E-mail: jacques.simard@crchul.ulaval.ca.
This work was supported by the Medical Research Council of Canada/Canadian Institutes of Health Research (Medical Research Council Group in Molecular Endocrinology), Endorecherche, and in part by grants (to J.-L.C.) awarded by FRSQ and INSERM (France).
* Recipient of a studentship from Medical Research Council.
✝ Senior Scientist from Le Fond de la Recherche en Sante du Quebec and currently chairholder of the Canada Research Chair in Oncogenetics funded by the Canada Research Chairs Program.
REFERENCES
- Liu KD, Gaffen SL, Goldsmith MA 1998 JAK/Stat signaling by cytokine receptors. Curr Opin Immunol 10: 271–278
- Darnell Jr JE 1997 Stats and gene regulation. Science 277:1630–1635
- Bromberg J, Darnell Jr JE 2000 The role of Stats in transcriptional control and their impact on cellular function. Oncogene 19:2468–2473
- Chatterjee-Kishore M, van den Akker F, Stark GR 2000 Association of Stats with relatives and friends. Trends Cell Biol 10:106–111
- Schmitt-Ney M, Doppler W, Ball RK, Groner B 1991 β-Casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol 11:3745–3755
- Wakao H, Schmitt-Ney M, Groner B 1992 Mammary gland-specific nuclear factor is present in lactating rodent and bovine mammary tissue and composed of a single polypeptide of 89 kDa. J Biol Chem 267: 16365–16370
- Welte T, Garimorth K, Philipp S, Doppler W 1994 Prolactin-dependent activation of a tyrosine phosphorylated DNA binding factor in mouse mammary epithelial cells. Mol Endocrinol 8:1091–1102
- Leonard WJ, O’Shea JJ 1998 Jaks and Stats: biological implications. Annu Rev Immunol 16:293–322
- Hoey T, Schindler U 1998 Stat structure and function in signaling. Curr Opin Genet Dev 8:582–587
- Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L 1995 Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA 92:8831–8835
- Moriggl R, Gouilleux-Gruart V, Jahne R, Berchtold S, Gartmann C, Liu X, Hennighausen L, Sotiropoulos A, Groner B, Gouilleux F 1996 Deletion of the carboxylterminal transactivation domain of MGF-Stat5 results in sustained DNA binding and a dominant negative phenotype. Mol Cell Biol 16:5691–5700
- Boucheron C, Dumon S, Santos SC, Moriggl R, Hennighausen L, Gisselbrecht S, Gouilleux F 1998 A single amino acid in the DNA binding regions of Stat5A and Stat5B confers distinct DNA binding specificities. J Biol Chem 273:33936–33941
- Verdier F, Rabionet R, Gouilleux F, Beisenherz-Huss C, Varlet P, Muller O, Mayeux P, Lacombe C, Gisselbrecht S, Chretien S 1998 A sequence of the CIS gene promoter interacts preferentially with two associated Stat5A dimers: a distinct biochemical difference between Stat5A and Stat5B. Mol Cell Biol 18:5852–5860
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L 1997 Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11:179–186
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW 1997 Requirement of Stat5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94:7239–7244
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN 1998 Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841–850
- Davey HW, Park SH, Grattan DR, McLachlan MJ, Waxman DJ 1999 Stat5b-deficient mice are growth hormone pulse-resistant. Role of Stat5b in sex-specific liver p450 expression. J Biol Chem 274:35331–3536
- Fisher B, Costantino C, ER R, Fisher D, Wickerham L, Cronin W, Contributors N 1994 Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 86:527–537
- Tsai MJ, O’Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486
- Di Croce L, Okret S, Kersten S, Gustafsson J-A, Parker M, Wahli W, Beato M 1999 Steroid and nuclear receptors. EMBO J 18:6201–6210
- Dumler I, Kopmann A, Wagner K, Mayboroda OA, Jerke U, Dietz R, Haller H, Gulba DC 1999 Urokinase induces activation and formation of Stat4 and Stat1-Stat2 complexes in human vascular smooth muscle cells. J Biol Chem 274:24059–24065
- Look DC, Pelletier MR, Tidwell RM, Roswit WT, Holtzman MJ 1995 Stat1 depends on transcriptional synergy with Sp1. J Biol Chem 270:30264–30267
- Ohmori Y, Schreiber RD, Hamilton TA 1997 Synergy between interferon-γ and tumor necrosis factor-α in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor κB. J Biol Chem 272:14899–14907
- Zhang JJ, Vinkemeier U, Gu W, Chakravarti D, Horvath CM, Darnell Jr JE 1996 Two contact regions between Stat1 and CBP/p300 in interferon γ signaling. Proc Natl Acad Sci USA 93:15092–15096
- Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D’Andrea A, Livingston DM 1996 Cooperation of Stat2 and p300/CBP in signalling induced by interferon-α. Nature 383:344–347
- Pfitzner E, Jahne R, Wissler M, Stoecklin E, Groner B 1998 p300/CREB-binding protein enhances the prolactin-mediated transcriptional induction through direct interaction with the transactivation domain of Stat5, but does not participate in the Stat5-mediated suppression of the glucocorticoid response. Mol Endocrinol 12: 1582–1593
- Gingras S, Simard J, Groner B, Pfitzner E 1999 p300/CBP is required for transcriptional induction by interleukin-4 and interacts with Stat6. Nucleic Acids Res 27:2722–2729
- Zhou YC, Waxman DJ 1999 Stat5b down-regulates peroxisome proliferator-activated receptor α transcription by inhibition of ligand-independent activation function region-1 trans-activation domain. J Biol Chem 274:29874–29882
- Zhou YC, Waxman DJ 1999 Cross-talk between Janus kinase-signal transducer and activator of transcription (JAK-Stat) and peroxisome proliferator-activated receptor-α (PPARα) signaling pathways. Growth hormone inhibition of pparα transcriptional activity mediated by stat5b. J Biol Chem 274:2672–2681
- Hocke GM, Barry D, Fey GH 1992 Synergistic action of interleukin-6 and glucocorticoids is mediated by the interleukin-6 response element of the rat α2 macroglobulin gene. Mol Cell Biol 12:2282–2294
- Stocklin E, Wissler M, Gouilleux F, Groner B 1996 Functional interactions between Stat5 and the glucocorticoid receptor. Nature 383:726–728
- Cella N, Groner B, Hynes NE 1998 Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells. Mol Cell Biol 18:1783–1792
- Stoecklin E, Wissler M, Moriggl R, Groner B 1997 Specific DNA binding of Stat5, but not of glucocorticoid receptor, is required for their functional coop ration in the regulation of gene transcription. Mol Cell Biol 17: 6708–6716
- Lechner J, Welte T, Tomasi JK, Bruno P, Cairns C, Gustafsson J, Doppler W 1997 Promoter-dependent synergy between glucocorticoid receptor and Stat5 in the activation of β-casein gene transcription. J Biol Chem 272:20954–20960
- Stoecklin E, Wissler M, Schaetzle D, Pfitzner E, Groner B 1999 Interactions in the transcriptional regulation exerted by Stat5 and by members of the steroid hormone receptor family. J Steroid Biochem Mol Biol 69:195–204
- Bjormstrom L, Kilic E, Norman M, Parker MG, Sjoberg M 2001 Cross-talk between Stat5b and estrogen receptor-α and -β in mammary epithelial cells. J Mol Endocrinol 27:93–106
- Rosen JM, Wyszomierski SL, Hadsell D 1999 Regulation of milk protein gene expression. Annu Rev Nutr 19:407–436
- Shiu RP, Iwasiow BM 1985 Prolactin-inducible proteins in human breast cancer cells. J Biol Chem 260:11307–11313
- Haagensen Jr DE, Dilley WG, Mazoujian G, Wells Jr SA 1990 Review of GCDFP-15. An apocrine marker protein. Ann NY Acad Sci 586:161–173
- Silva JS, Leight GS, Haagensen Jr DE, Tallos PB, Cox EB, Dilley WG, Wells Jr SA 1982 Quantitation of response to therapy in patients with metastatic breast carcinoma by serial analysis of plasma gross cystic disease fluid protein and carcinoembryonic antigen. Cancer 49:1236–1242
- Sanchez LM, Vizoso F, Diez-Itza I, Lopez-Otin C 1992 Identification of the major protein components in breast secretions from women with benign and malignant breast diseases. Cancer Res 52:95–100
- Caputo E, Manco G, Mandrich L, Guardiola J 2000 A novel aspartyl proteinase from apocrine epithelia and breast tumors. J Biol Chem 275:7935–7941
- Murphy LC, Tsuyuki D, Myal Y, Shiu RP 1987 Isolation and sequencing of a cDNA clone for a prolactin-inducible protein (PIP). Regulation of PIP gene expression in the human breast cancer cell line, T-47D. J Biol Chem 262:15236–15241
- Simard J, Hatton AC, Labrie C, Dauvois S, Zhao HF, Haagensen DE, Labrie F 1989 Inhibitory effect of estrogens on GCDFP-15 mRNA levels and secretion in ZR-75-1 human breast cancer cells. Mol Endocrinol 3:694–702
- Blais Y, Sugimoto K, Carriere MC, Haagensen DE, Labrie F, Simard J 1994 Potent stimulatory effect of interleukin-1α on apolipoprotein D and gross cystic disease fluid protein-15 expression in human breast-cancer cells. Int J Cancer 59:400–407
- Blais Y, Gingras S, Haagensen DE, Labrie F, Simard J 1996 Interleukin-4 and interleukin-13 inhibit estrogeninduced breast cancer cell proliferation and stimulate GCDFP-15 expression in human breast cancer cells. Mol Cell Endocrinol 121:11–18
- Myal Y, Robinson DB, Iwasiow B, Tsuyuki D, Wong P, Shiu RP 1991 The prolactin-inducible protein (PIP/GCDFP-15) gene: cloning, structure and regulation. Mol Cell Endocrinol 80:165–175
- Langer SJ, Ostrowski MC 1988 Negative regulation of transcription in vitro by a glucocorticoid response element is mediated by a trans-acting factor. Mol Cell Biol 8:3872–2881
- Rose JK BJ 1982 Expression from cloned cDNA of cellsurface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell 30:753–762
- Mui AL, Wakao H, Kinoshita T, Kitamura T, Miyajima A 1996 Suppression of interleukin-3-induced gene expression by a C-terminal truncated Stat5: role of Stat5 in proliferation. EMBO J 15:2425–2433
- Gouilleux F, Wakao H, Mundt M, Groner B 1994 Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J 13:4361–4369
- Shuai K, Horvath CM, Huang LH, Qureshi SA, Cowburn D, Darnell Jr JE 1994 Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell 76:821–828
- Wang D, Stravopodis D, Teglund S, Kitazawa J, Ihle JN 1996 Naturally occurring dominant negative variants of Stat5. Mol Cell Biol 16:6141–6148
- Lubahn DB, Joseph DR, Sar M, Tan J, Higgs HN, Larson RE, French FS, Wilson EM 1988 The human androgen receptor: complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol 2:1265–1275
- Wang Q, Ghadessy FJ, Trounson A, de Kretser D, McLachlan R, Ng SC, Yong EL 1998 Azoospermia associated with a mutation in the ligand-binding domain of an androgen receptor displaying normal ligand binding, but defective trans-activation. J Clin Endocrinol Metab 83:4303–4309
- Lundberg Giwercman Y, Nikoshkov A, Lindsten K, Bystrom B, Pousette A, Chibalin AV, Arvidsson S, Tiulpakov A, Semitcheva TV, Peterkova V, Hagenfeldt K, Ritzen EM, Wedell A 1998 Functional characterisation of mutations in the ligand-binding domain of the androgen receptor gene in patients with androgen insensitivity syndrome. Hum Genet 103:529–531
- Nazareth LV, Stenoien DL, Bingman III WE, James AJ, Wu C, Zhang Y, Edwards DP, Mancini M, Marcelli M, Lamb DJ, Weigel NL 1999 A C619Y mutation in the human androgen receptor causes inactivation and mislocalization of the receptor with concomitant sequestration of SRC-1 (steroid receptor coactivator 1). Mol Endocrinol 13:2065–2075
- Bowman T, Garcia R, Turkson J, Jove R 2000 Stats in oncogenesis. Oncogene 19:2474–2488
- Shuai K 2000 Modulation of Stat signaling by Stat-interacting proteins. Oncogene 19:2638–2644
- Luo G, Yu-Lee L 1997 Transcriptional inhibition by Stat5. Differential activities at growth-related vs. differentiation-specific promoters. J Biol Chem 272:26841–26849
- Nelson CC, Hendy SC, Shukin RJ, Cheng H, Bruchovsky N, Koop BF, Rennie PS 1999 Determinants of DNA sequence specificity of the androgen, progesterone, and glucocorticoid receptors: evidence for differential steroid receptor response elements. Mol Endocrinol 13:2090–2107
- Meinke A, Barahmand-Pour F, Wohrl S, Stoiber D, Decker T 1996 Activation of different Stat5 isoforms contributes to cell-type-restricted signaling in response to interferons. Mol Cell Biol 16:6937–6944
- Wennbo H, Tornell J 2000 The role of prolactin and growth hormone in breast cancer. Oncogene 19:1072–1076
- Biswas R, Vonderhaar BK 1987 Role of serum in the prolactin responsiveness of MCF-7 human breast cancer cells in long-term tissue culture. Cancer Res 47:3509–3514
- Kiss R, de Launoit Y, L’Hermite-Baleriaux M, L’Hermite M, Paridaens RJ, Danguy AJ, Pasteels JL 1987 Effect of prolactin and estradiol on cell proliferation in the u erus and the MXT mouse mammary neoplasm. J Natl Cancer Inst 78:993–998
- Fels E 1944 Treatment of breast cancer with testosterone propionate. A preliminary report. J Clin Endocrinol 4:121–125
- Kennedy B 1958 Fluoxymesterone therapy in treatment of advanced breast cancer. N Engl J Med 259:673–675
- Cooperative Breast Cancer Group 1964 Testosterone propionate therapy of breast cancer. J Am Med Assoc 188:1069–1072
- Gordan GS, Halden A, Horn Y, Fuery JJ, Parsons RJ, Walter RM 1973 Calusterone (7β,17α-dimethyltestosterone) as primary and secondary therapy of advanced breast cancer. Oncology 28:138–146
- Tormey DC, Lippman ME, Edwards BK, Cassidy JG 1983 Evaluation of tamoxifen doses with and without fluoxymesterone in advanced breast cancer. Ann Intern Med 98:139–144
- Ingle JN, Twito DI, Schaid DJ, Cullinan SA, Krook JE, Mailliard JA, Tschetter LK, Long HJ, Gerstner JG, Windschitl HE, Levitt R, Pfeifle DM 1991 Combination hormonal therapy with tamoxifen plus fluoxymesterone vs. tamoxifen alone in postmenopausal women with metastatic breast cancer. An updated analysis. Cancer 67:886–891
- Trams G, Maass H 1977 Specific binding of estradiol and dihydrotestosterone in human mammary cancers. Cancer Res 37:258–261
- Allegra JC, Lippman ME, Thompson EB, Simon R, Green L, Barlock A, Green L, Huff KK, Do HM, Aitken SC 1979 Distribution, frequency and quantitative analysis of estrogen, progesterone, androgen and glucocorticoid receptors in human breast cancer. Cancer Res 39:1447–1454
- Bryan RM, Mercer RJ, Bennett RC, Rennie GC, Lie TH, Morgan FJ 1984 Androgen receptors in breast cancer. Cancer 54:2436–2440
- Miller WR, Telford J, Dixon JM, Hawkins RA 1985 Androgen receptor activity in human breast cancer and its relationship with estrogen and progesterone receptor activity. Eur J Cancer Clin Oncol 21:539–542
- Suzuki T, Darnel AD, Akahira JI, Ariga N, Ogawa S, Kaneko C, Takeyama J, Moriya T, Sasano H 2001 5α-Reductases in human breast carcinoma: possible modulator of in situ androgenic actions. J Clin Endocrinol Metab 86:2250–2257
- Labrie F, Luu-The V, Labrie C, Simard J 2001 DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol 22:185–212
- Labrie F, Simard J, de Launoit Y, Poulin R, Theriault C, Dumont M, Dauvois S, Martel C, Li SM 1992 Androgens and breast cancer. Cancer Detect Prev 16:31–38
- Simard J, Gingras S 2001 Crucial role of cytokines in sex steroid formation in normal and tumoral tissues. Mol Cell Endocrinol 171:25–40
- Luo S, Sourla A, Labrie C, Belanger A, Labrie F 1997 Combined effects of dehydroepiandrosterone and EM-800 on bone mass, serum lipids, and the development of dimethylbenz(A)anthracene-induced mammary carcinoma in the rat. Endocrinology 138:4435–4444
- Couillard S, Labrie C, Belanger A, Candas B, Pouliot F, Labrie F 1998 Effect of dehydroepiandrosterone and the antiestrogen EM-800 on growth of human ZR-75-1 breast cancer xenografts. J Natl Cancer Inst 90:772–778
- Poulin R, Baker D, Labrie F 1988 Androgens inhibit basal and estrogen-induced cell proliferation in the ZR-74 human breast cancer cell line. Breast Cancer Res Treat 12:213–225
- Hackenberg R, Luttchens S, Hofmann J, Kunzmann R, Holzel F, Schulz KD 1991 Androgen sensitivity of the new human breast cancer cell line MFM-223. Cancer Res 51:5722–5727
- Lapointe J, Fournier A, Richard V, Labrie C 1999 Androgens down-regulate bcl-2 protooncogene expression in ZR-75-1 human breast cancer cells. Endocrinology 140:416–421
- Lapointe J, Labrie C 2001 Role of the cyclin-dependent kinase inhibitor p27Kip1 in androgen-induced inhibition of CAMA-1 breast cancer cell proliferation. Endocrinology 142:4331–4338
- Dauvois S, Geng CS, Levesque C, Merand Y, Labrie F 1991 Additive inhibitory effects of an androgen and the antiestrogen EM-170 on estradiol-stimulated growth of human ZR-75-1 breast tumors in athymic mice. Cancer Res 51:3131–3135
- de Launoit Y, Dauvois S, Dufour M, Simard J, Labrie F 1991 Inhibition of cell cycle kinetics and proliferation by the androgen 5α-dihydrotestosterone and antiestrogen N,n-butyl-N-methyl-11-[16`α-chloro-3`,17β-dihydroxyestra-1`,3`,5`-(10`)triene-7`α-yl]undecanamide in human breast cancer ZR-75-1 cells. Cancer Res 51:2797–2802
- Poulin R, Simard J, Labrie C, Petitclerc L, Dumont M, Lagace L, Labrie F 1989 Down-regulation of estrogen receptors by androgens in the ZR-75-1 human breast cancer cell line. Endocrinology 125:392–399
- Simard J, de Launoit Y, Haagensen DE, Labrie F 1992 Additive stimulatory action of glucocorticoids and androgens on basal and estrogen-repressed apolipoprotein-D messenger ribonucleic acid levels and secretion in human breast cancer cells. Endocrinology 130:1115–1121
- Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT 1989 Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with α-lactalbumin. Hum Pathol 20:281–287
- Monteagudo C, Merino MJ, LaPorte N, Neumann RD 1991 Value of gross cystic disease fluid protein-15 in distinguishing metastatic breast carcinomas among poorly differentiated neoplasms involving the ovary. Hum Pathol 22:368–372
- Dumont M, Dauvois S, Simard J, Garcia T, Schachter B, Labrie F 1989 Antagonism between estrogens and androgens on GCDFP-15 gene expression in ZR-75-1 cells and correlation between GCDFP-15 and estrogen as well as progesterone receptor expression in human breast cancer. J Steroid Biochem 34:397–402
- Dauvois S, Simard J, Dumont M, Haagensen DE, Labrie F 1990 Opposite effects of estrogen and the progestin R5020 on cell proliferation and GCDFP-15 expression in ZR-75-1 human breast cancer cells. Mol Cell Endocrinol 73:171–178
- Gaubin M, Autiero M, Basmaciogullari S, Metivier D, Mis hal Z, Culerrier R, Oudin A, Guardiola J, Piatier-Tonneau D 1999 Potent inhibition of CD4/TCR-mediated T cell apoptosis by a CD4-binding glycoprotein secreted from breast tumor and seminal vesicle cells. J Immunol 162:2631–2638
- Gingras S, Moriggl R, Groner B, Simard J 1999 Induction of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase type 1 gene transcription in human breast cancer cell lines and in normal mammary epithelial cells by interleukin-4 and interleukin-13. Mol Endocrinol 13:66–81